COVID-19 Test
For Healthcare Professionals
COVID-19 testing overview
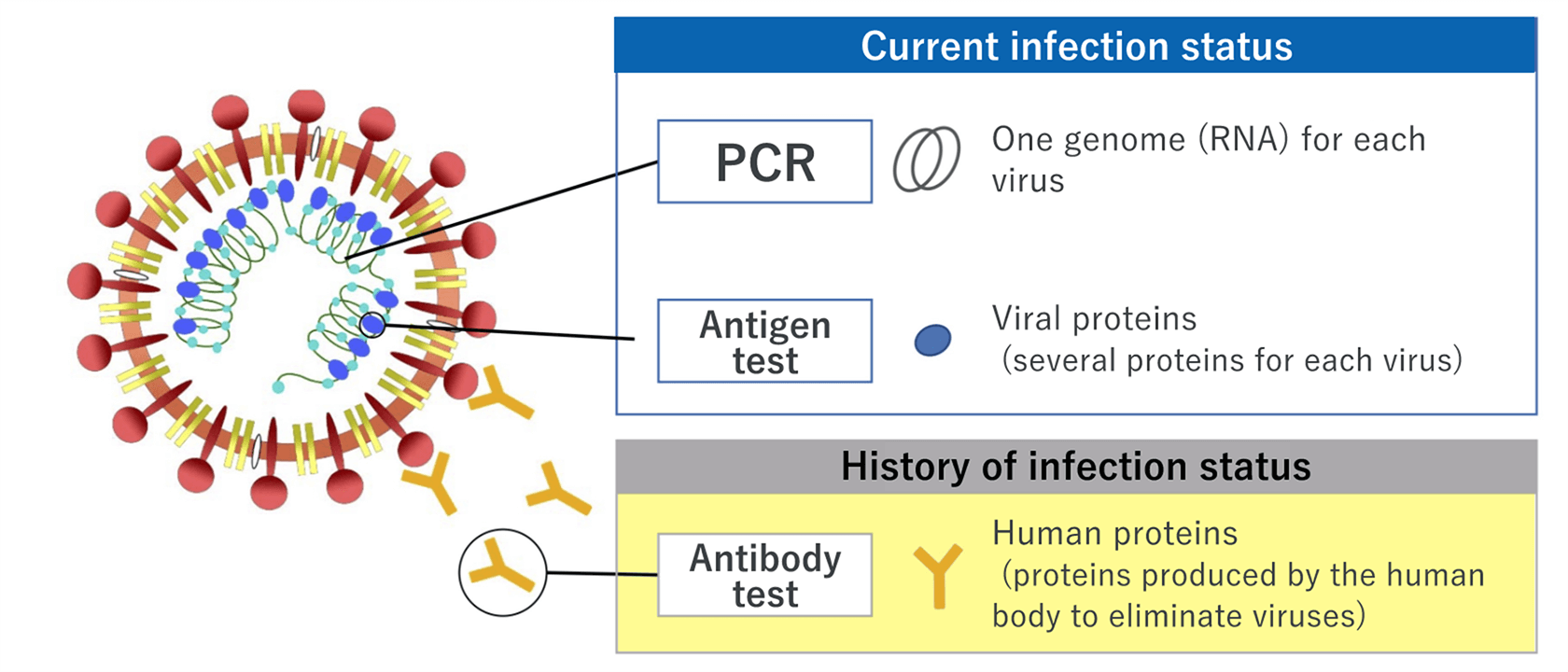
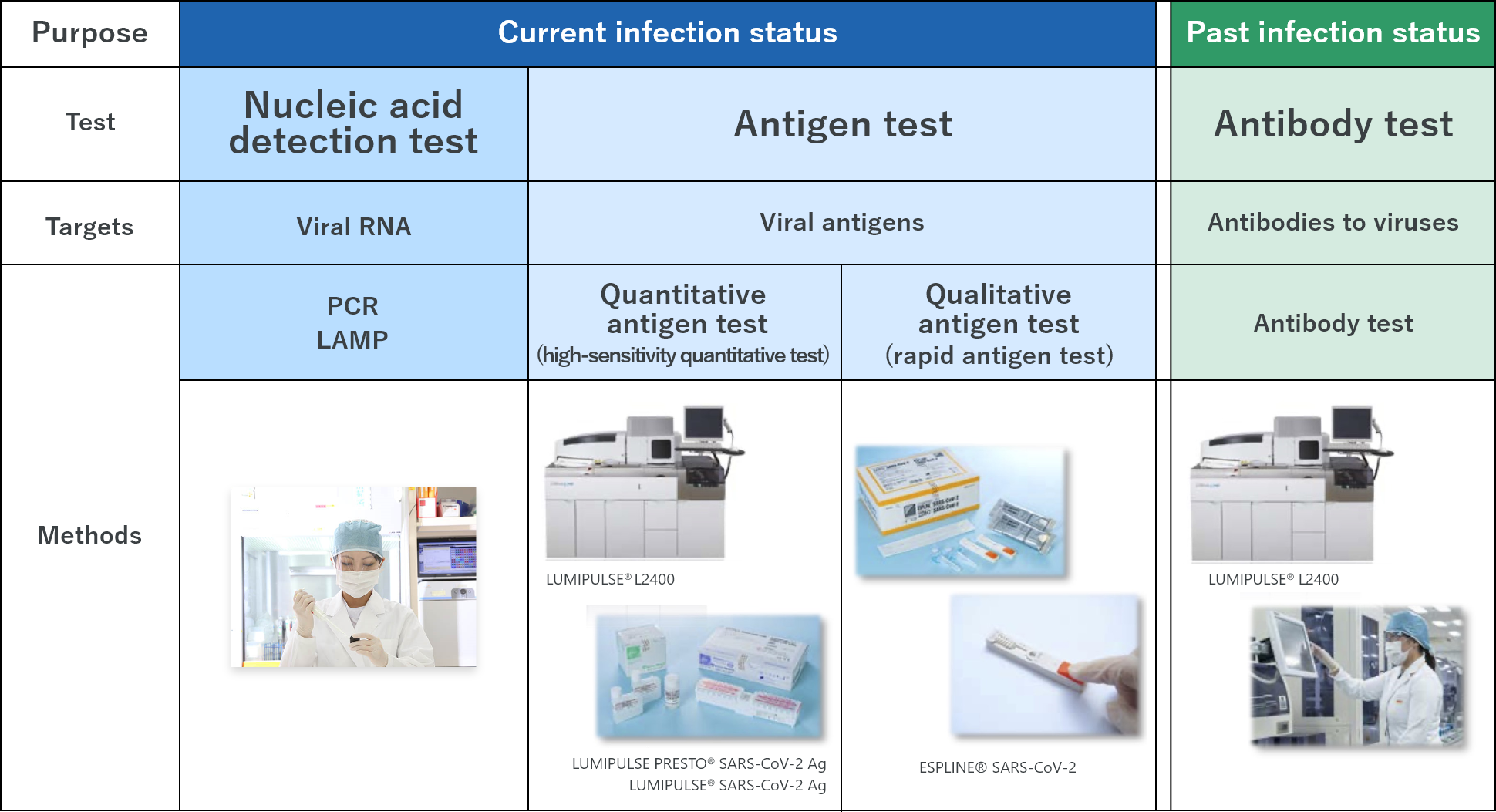
There are mainly three types of COVID-19 tests: nucleic acid detection tests, antigen tests, and antibody tests. Here, we explain the differences, characteristics, advantages and disadvantages of each test.
Types and characteristics of tests
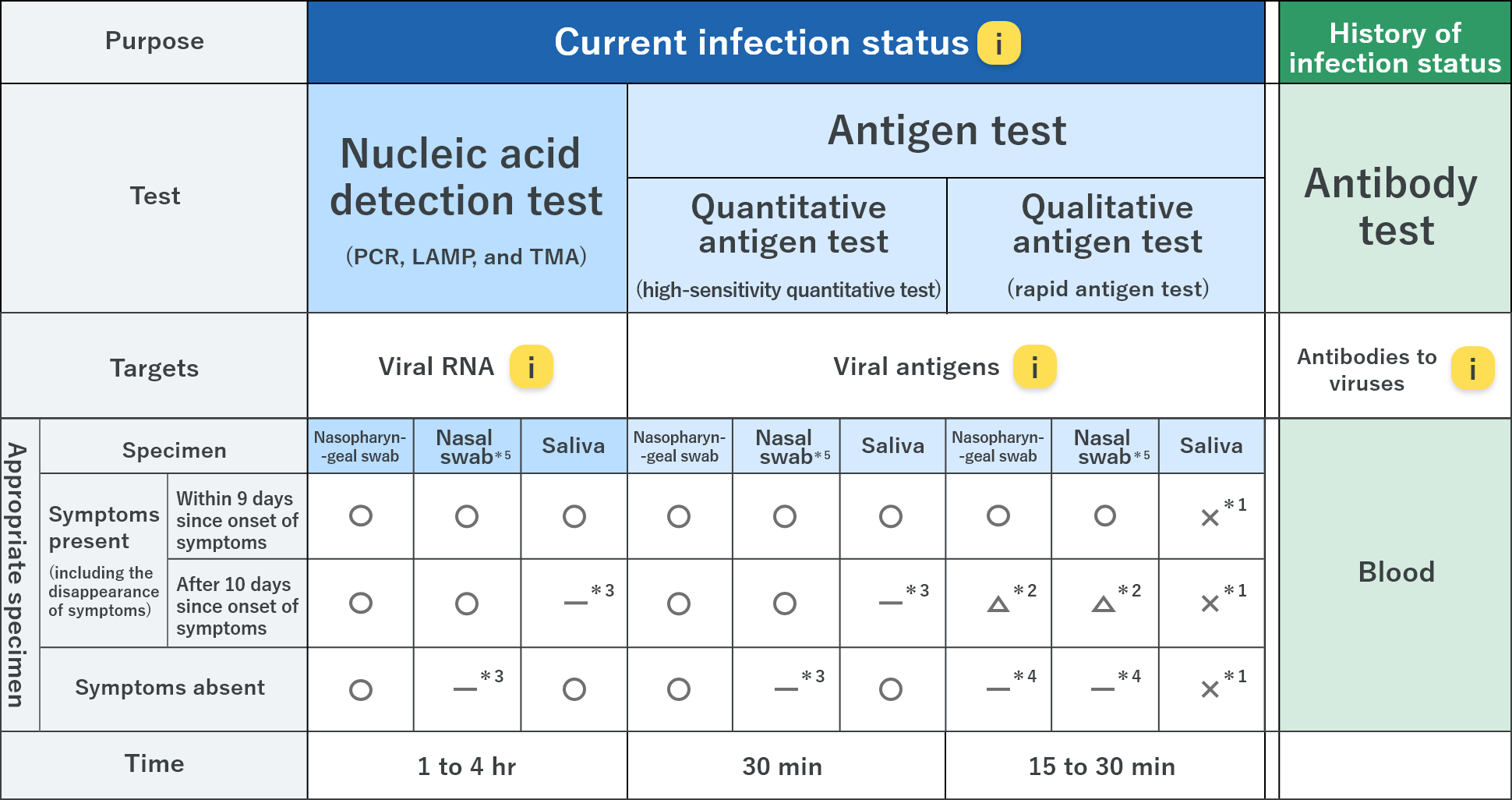

*1 Usage for those with symptoms is under investigation; usage for those without symptoms will be investigated (×)
*2 Enable to use; however, in case of a negative result, nucleic acid detection test or quantitative antigen test are recommended according to the clinical appearance (△)
*3 Not recommended
*4 Although not recommended for definitive diagnosis, it can be used for screening when a number of testing is needed for medical facilities or nursing homes in the areas where the infection has spread; infection control
measures should be continued even if the results are negative, and the results should be confirmed by a nucleic acid detection test or high-sensitivity quantitative test based on the physician’s decision if the results are positive.
*5 Considered to be a useful sample; however, further investigation is needed.
Nucleic acid detection test – the widely used testing method, which includes PCR test that can detect viral RNA of the new coronavirus (SARS-CoV-2)
Nucleic acid detection test is a method of detecting the infection of SARS-CoV-2 by viral RNA. Generally, the polymerase chain reaction (PCR) method that amplifies the viral RNA is used. Several methods of amplification, including loop-mediated isothermal amplification (LAMP) and transcript-mediated amplification (TMA) as simple and fast methods, are also available. Nasopharyngeal swabs, nasal swabs, or saliva can be used.
■ Features of PCR test
- can detect even with a small amount of virus; it is available for both symptomatic and asymptomatic patients
-
performance varies depending on reagents and methods; thus, appropriate reagents should be used based on the situation and environment1,2)
- specimens that cannot be isolated and cultured because they are not considered to be infectious might result in positive samples; other clinical findings should be taken into account for clinical decision making
- has a risk of contamination (effect of amplified products of other specimens, which result in false positives) and PCR inhibitory substances ― quality control is important
References
1) MacKay MJ, et al. Nat Biotechnol. 2020; 38: 1021–1024.
2) 宮地 勇人. 厚生労働省委託事業「新型コロナウイルス感染症のPCR 検査等にかかる精度管理調査業務」報告書 https://www.mhlw.go.jp/content/000769978.pdf.
Accessed June 28, 2021. (Available in JAPANESE)
Antigen test ― gives results within a short time; two types of techniques, high-sensitivity quantitative test and rapid antigen test, are available
Antigen test is a method of detecting the viral antigen of SARS-CoV-2. There are two types of tests: one is the high-sensitivity quantitative antigen test, and the other is the rapid antigen test.
The rapid antigen test can
be used for point-of-care testing (POCT). Currently, nasopharyngeal or nasal swabs can be used, while saliva cannot be used. Nasal swabs can be collected by patients themselves under the supervision of medical personnel. However,
if there is a need for a rapid antigen test in a facility where resident medical professionals are absent, it can be performed under the supervision of staff who understand the precautions for specimen collection. H.U. Group’s
high-sensitivity quantitative antigen test reagent, LUMIPULSE SARS-CoV-2 Ag, can be used with saliva that can be collected by the patient.
■ Features of the high-sensitivity quantitative antigen test
- can detect even a small amount of virus; it is available for both symptomatic and asymptomatic patients
- can give a result within ~30 minutes
- has the same level of detection accuracy as some nucleic acid detection test, and can be used for definitive diagnosis
- enables a fully automated system that allows for high-throughput (mass processing) testing using specialized equipment
- can be performed using specialized equipment available in general laboratories
- may require retesting due to non-specific reaction in some cases; in such cases, retests using supernatant after centrifugation or nucleic acid detection test should be performed
■ Features of the rapid antigen test
- could give a false-negative result (a negative result despite being infected) when the viral load is not above a certain level
- can give a result within 15 to 30 minutes
- does not often require special equipment and can give a result on site
- is effective when a large number of tests are required, such as in areas where the infection has spread
- is useful for those who are at risk of spreading the infection to others and have sufficient viral load to be detectable
- is not recommended for screening of asymptomatic patients; it can be used when a quick response is required, although a follow-up test (i.e., nucleic acid detection test) should be performed in consideration of the low reliability
Antibody test ― indicates the history of the SARS-CoV-2 infection status
Antibody test is a method of identifying a past infection of SARS-CoV-2 by detecting antibodies in blood. Antibody tests show positive results 1 to 3 weeks after infection; thus, antibody tests may not show a current infection status. Testing of various antibodies are available for different targets depending on the purpose of the test.
■ Features of the antibody test
- uses blood samples
- can indicate a history of SARS-CoV-2 infection
- can detect antibodies 1 to 3 weeks after infection (may not show a current SARS-CoV-2 infection status)
- IgM is expected to increase within ~1 to 2 weeks after infection, and then decrease.
- IgG is expected to increase at about the same time as IgM after infection, and is then maintained for a period of time
- Test results (levels of antibodies to SARS-CoV-2) are expected to increase within a few weeks after vaccination
- Antibodies to nucleocapsid proteins are not expected to increase with the currently available SARS-CoV-2 vaccines in Japan (as of August 1, 2021)
- Antibodies to the spike proteins are expected to increase after vaccination with currently available vaccines in Japan, giving an indistinguishable result from that of a history of infection
- There is no approved reagent in Japan; it is available only for research use (as of August 1, 2021)
Reference information – types of antibodies that can be used for antibody testing and their characteristics
| Types of antibodies | Characteristics |
|---|---|
| IgG antibodies to the spike (S) protein of SARS-CoV-2. | Intended for use as an aid in identifying individuals with adaptive immune responses to SARS-CoV-2; recent or prior infection can be confirmed. This assay can be used to detect antibody responses induced by currently available SARS-CoV-2 vaccines in Japan. |
| Antibodies to the nucleocapsid (N) protein of SARS-CoV-2. | Intended for use as an aid in identifying individuals with adaptive immune responses to SARS-CoV-2; recent or prior infection can be confirmed. This assay can be used to differentiate between antibodies elicited due to natural infection or vaccination. However, this assay cannot be used to detect antibody responses induced by currently available SARS-CoV-2 vaccines in Japan. |
| IgM antibodies to the spike (S) protein of SARS-CoV-2. | Intended for use as an aid in identifying individuals with adaptive immune responses to SARS-CoV-2, used with the IgG antibody test. IgM antibodies are produced at approximately the same time as IgG antibodies after SARS-CoV-2 infection. |
Differences between samples
Nasopharyngeal swabs, nasal swabs, or saliva can be used for PCR tests (nucleic acid detection test).
SARS-CoV-2 is often transmitted through the upper respiratory tract; thus, using nasopharyngeal swabs is considered as a
standard method and reliable option for testing early infection. Thorough infection control measures should be taken when healthcare professionals collect samples due to high risk of exposure to droplets.
The samples can
be collected by the patients in the case of saliva or nasal swabs, which may help reduce the risk of infection to healthcare professionals. Caution is needed when using saliva; using saliva collected after eating, drinking,
gargling, or brushing teeth may affect the detection of the virus. Also, saliva samples collected 10 days after the onset of symptoms is not recommended for testing; the sensitivity with these saliva samples decreases for both
nucleic acid detection and antigen tests compared to that with nasopharyngeal swab samples, and the risk of false negative results increases.
Sensitivity with nasal swab samples may be lower than that with nasopharyngeal
swab samples; however, it is considered to be useful in terms of practicality and less risk of infection to healthcare professionals.
Please refer to the Ministry of Health, Labour and Welfare, et al. New Coronavirus Infectious Disease (COVID-19) Pathogen Testing Guideline Version 4

Types of COVID-19 tests and their suitable usage
Since different tests have different characteristics, the test to be performed depends on the situation. It is important to perform the tests in accordance to the situation at hand.
■ Examples of types of tests under different scenarios
- A person who claimed symptoms such as fever, visited a medical facility, and was suspected to have COVID-19.
Nucleic acid detection test
High-sensitivity quantitative antigen test
Rapid antigen test(within 9 days since onset of symptoms)

- To check the infection status of persons in close contact (including asymptomatic persons) with patients known to have COVID-19.
- Cluster occurred in a medical facility, and there is a need for screening.
Nucleic acid detection test
High-sensitivity quantitative antigen test
Rapid antigen test

- To perform rapid testing of symptomatic patients in high-risk facilities.

Rapid antigen test

- To perform rapid and highly sensitive test to check for COVID-19 in a limited environment for a limited number of staff members.
High-sensitivity quantitative antigen test

- Need proof of negative results before overseas business trip.

Nucleic acid detection test
High-sensitivity quantitative antigen test / rapid antigen test
(depending on the country)

- To determine the infection status in the past.
- To determine if antibody titer is elevated due to vaccination.
Antibody test

Our efforts for the fight against COVID-19
(1) Introduction and development of tests for detecting SARS-CoV-2
In January 2020, before Japan faced the impact of the COVID-19 crisis, we established the PCR test system and launched development of the antigen test system; we were the first company to initiate the PCR testing service for SARS-CoV-2
in Japan. In May 2020, our rapid antigen test was approved as the first rapid antigen test in Japan; in June 2020, our high-sensitivity quantitative antigen test was approved. Since June 2020, SRL Inc. has been performing contract
services for antibody testing, and since March 2021, FUJIREBIO Inc. has been selling antibody test reagents for research purposes.
In order to deliver the most appropriate test for each situation, we have developed a wide
range of testing systems and been supplying reagents. In particular, LUMIPULSE SARS-CoV-2 Ag was the first in its class as a high-sensitivity quantitative antigen test in the world; it is a highly sensitive and high-throughput
quantitative antigen test. Saliva can be used, which helps reduce the risk of infection and the burden of sample collection and measurement on medical professionals. The high throughput of our high-sensitivity quantitative
test is a powerful screening method; the sensitivity is comparable to that of nucleic acid detection tests, and it can be used for asymptomatic individuals.
Type of tests (Our services/products)

(2) Pursuit of test accuracy
SRL Inc. has more than 50 years of experience in clinical testing, and has been providing the highest standards for testing on consignment from medical facilities. In addition, we believe that providing stable tests is vital. To
date, we have rigorously evaluated a number of reagents for testing SARS-CoV-2, and have selected reagents with proved performance and availability for daily testing. We also monitor the test results periodically to confirm
the accuracy of the daily tests.
ESPLINE SARS-CoV-2 Ag developed by FUJIREBIO Inc. is a test reagent that uses antibodies that bind to the nucleocapsid protein of the virus and can provide test results within 30 minutes with high sensitivity. ESPLINE
SARS-CoV-2 Ag has been compared with other products by third parties. In order to compare testing methods, evaluating under the same conditions is important because the condition of sampling (e.g., time from onset of symptoms
to collection of samples) and different types of samples could significantly impact the test results.

Yamayoshi et al. of the Institute of Medical Science, the University of Tokyo, reported the sensitivities of several rapid antigen tests using SARS-CoV-2 isolates and several types of COVID-19 patient specimens. The report showed sufficient
sensitivity of ESPLINE SARS-CoV-2 Ag to detect viral antigens in almost all specimens which possessed infectious potential1). Moreover, the sensitivity of ESPLINE SARS-CoV-2 Ag was comparable to that of other companies’
products. Cubas-Atienzar et al. reported ESPLINE SARS-CoV-2 Ag showed one of the highest sensitivity compared to nineteen rapid antigen test methods using direct culture supernatant2). FIND, a global non-profit organization,
reported the clinical sensitivity of ESPLINE SARS-CoV-2 Ag of 78.6%, and clinical sensitivity with Ct (number of cycles when the RT-PCR showed positive result) ≤25 of 92%3). The results were comparable to those of other
companies’ products that were evaluated by FIND.
Some false positive results have been reported in ESPLINE SARS-CoV-2 Ag; in some cases, these results were due to inappropriate usage. Thus, we are working to improve awareness of appropriate use through providing videos of
how to use the tests and posting answers for frequently asked questions on the website of FUJIREBIO Inc.
References
1) Yamayoshi S, et al. Viruses 2020; 12: 1420.
2) Cubas-Atienzar AI, et al. Scientific Reports volume 11, Article number: 18313 (2021)
3) FIND. https://www.finddx.org/sarscov2-eval-antigen/ Accessed June 1, 2021.
(3) Enhancement of domestic testing
Our group company was the first private testing company to provide contract testing for COVID-19. Since then, we have been providing almost all types of tests, including nucleic acid detection tests such as PCR tests, antigen tests, and antibody tests,
on a 365-day basis throughout Japan. For PCR tests and high-sensitivity quantitative antigen tests, we have a capacity of more than 30,000 tests per day nationwide.
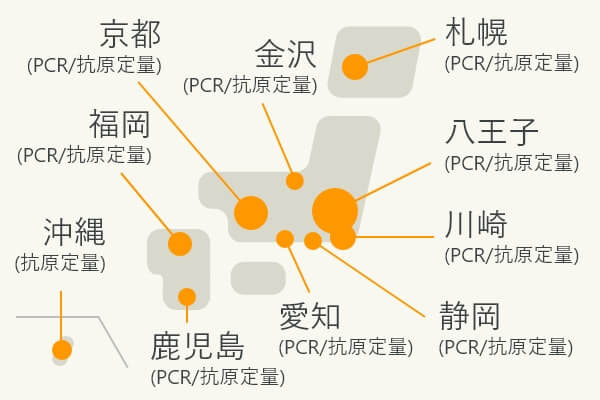 Nationwide test centers
Nationwide test centers (Establishing a nationwide testing system centered on the Hachioji Laboratory)
Our high-sensitivity quantitative antigen test reagents are available in automated systems to meet various needs. Our products are widely used at university hospitals, testing centers, and airport quarantines. Currently, a total
of more than 50 high-sensitivity quantitative antigen testing systems are in operation at eight airports in Japan.
The high-sensitivity quantitative antigen test has high accuracy while maintaining rapid results. However,
the positive predictive value tends to be lower when testing asymptomatic individuals. Thus, to improve accuracy, we recommend a two-step screening strategy with an initial high-sensitivity quantitative antigen test followed
by confirmatory nucleic acid detection test in specimen that are considered as “inconclusive” with the initial high-sensitivity quantitative test to reduce the risk of false positives4). This strategy is expected
to enable rapid and highly accurate testing. In fact, this two-step screening strategy is used in cases of airport quarantine to ensure rapid and safe border control measures.
Reference
4) Yokota I, et al. Lancet Microbe. 2021 May 19. doi: 10.1016/S2666-5247(21)00092-6.
 Inspection scene at the airport quarantine station
Inspection scene at the airport quarantine station
(4) Countermeasures for mutation and further research
■ For reliability
Even before the emergence of variants that affect viral replication and immunity as we know today, mutations that might affect test results had been reported.
In the nucleic acid detection test, mutations in the primer binding
site or probe binding site may result in false negatives. Thus, we have been evaluating routinely the effect of SARS-CoV-2 mutations on the test results by sequence analyses using next-generation sequencers and monitoring public
database and publications.
Mutations may affect antigen test results as well. Nucleic acid mutations do not always lead to amino acid mutations; antigen tests may be less susceptible to mutation than nucleic acid detection tests. On the other hand, amino acid changes in the antibody
binding site may result in false negatives. Thus, in the ESPLINE SARS-CoV-2 Ag (rapid antigen test reagent) and the LUMIPULSE SARS-CoV-2 Ag (high-sensitivity quantitative antigen test reagent), combination of antibodies that bind
to different regions of the antigen would reduce the risk of false negatives. In addition, as with the nucleic acid detection test, we monitor routinely for mutations in the sites where antibodies bind, as well as the impact of
mutations on the binding affinity of antibodies. In fact, at airport quarantine, variants brought in from outside Japan have been detected , thereby allowing for the smooth isolation procedures of those with infection. According
to the published report, those antigen test methods are useful for several variants5-9).
References
5) Kontogianni K, et al. J Infect. 2021; 83: e1-e4.
6) Hirotsu Y, et al. J Infect. 2021; 82: 276-316.
7) Loconsole D, et al. Microbiol Resour Announc. 2021; 10: e01487-21.
8) Loconsole D, et al. Clin Microbiol
Infect. 2021 May 10; S1198-743X(21)00228-7.
9) Caputo V, et al. Int J Infect Dis. 2021; 108: 187-189.
(5) Responding to the actual crisis
Since around December 2020, several mutations that might change the characteristics of the SARS-CoV-2 have been reported; mutations includes S:N501Y and S:L452R which are known to be highly infectious, and S:E484K which has been reported
to reduce the efficacy of the vaccine. As different mutations are reported daily, our continued mission has been to introduce PCR / sequence analysis technology to identify variants, and contribute to understanding how widely variants
are spread. Also, we are continuously monitoring the sensitivity of antigen tests as well as mutations that may affect the sensitivity of antigen tests. Currently, our products have little effect on the sensitivity to the various
variants that have been identified. At airport quarantine, our products can detect variants brought in from outside Japan, contributing to the early isolation of those with infection.
(6) Preparing for a future crisis

With the expectation of new variants continuously occurring in the future, we believe that it is extremely important to monitor mutations which might affect the testing and improve testing techniques which detect the variant correctly. We will continue our own research and will check and improve the PCR and antigen tests provided by the H.U. Group on a daily basis to detect any new variants.
(7) Social Contribution
We, at H.U. Group donated "ESPLINE SARS-CoV-2" the rapid antigen test kit for new coronaviruses to Nepal in September 2020 and to Pakistan in May 2021. In July 2021, we also donated the kit to the Ministry of Education, Culture, Sports, Science and Technology
(MEXT) for use in universities and other educational institutions to prevent the spread of infection.
The H.U. Group is working together to prevent the spread of new coronavirus infection.
Q&A for COVID-19 tests
A
Nucleic acid detection test, including PCR test, is a highly sensitive testing method. This test is often performed in a special laboratory because it requires special equipment. The performance varies depending on differences
in factors of each facility: differences in reagents used, variation in inhibitors depending on the reagents, and risks of contamination; therefore, quality control is important to obtain accurate results. Caution is needed
when using this test for detecting non-infectious viruses that are present even after individuals have recovered from infection.
There are two types of antigen tests: the high-sensitivity quantitative antigen test and
rapid antigen test. The high-sensitivity quantitative antigen test has the same detection accuracy as the PCR test except in cases of low viral load when infectivity is often low. The results can be obtained within as little
as 30 minutes. The high-sensitivity quantitative antigen test can be used for the definitive diagnosis of COVID-19. Also, it can be used for screening both symptomatic and asymptomatic individuals as with the nucleic acid
detection test. In addition, the use of the fully automated system allows for the sequential processing of a number of tests. Thus, it is used at airport quarantine, where results of a large number of tests are required
within a short period of time and at medical facilities that test a large number of patients.
Rapid antigen test is a method that often does not require special techniques or equipment and can provide results on-site
in 15-30 minutes. It is useful in clinics with a limited number of clinical laboratory specialists, in time-sensitive emergency medical situations, in medical facilities in areas where the infection is spreading, and in
elderly care facilities for testing asymptomatic individuals. Since each test has different characteristics, the most appropriate test should be used based on the situation.
In both tests, a false negative result might
occur at the early stages of infection because of insufficient virus load. Therefore, when in doubt, frequent testing should be performed. The antigen test, which is suitable for frequent testing due to its ease of use,
can be used to reduce the risk of missing detection at the early stages of infection.
On the other hand, automation might be necessary when performing PCR tests frequently due to the increase in contamination risks.
A
When viral load is insufficient or samples are not collected properly, the virus may not be detected even if the individual is infected. In particular, saliva samples are considered to be affected by smoking, eating, drinking, and mouthwash, so it is necessary to advise individuals not to smoke, eat, drink, or brush their teeth for at least 30 minutes prior to sample collection. In addition, various inhibitors may affect the test results, depending on the sample.
A
The H.U. Group offers PCR, antigen tests (high-sensitivity quantitative antigen test and rapid antigen test), and antibody test. For PCR test, among the various in vitro diagnostics, we select and use reagents that demonstrate
sufficient performance in measurements using actual samples after conducting in-house investigations. It has been reported that the performance of PCR tests varies depending on the test reagents. As we have found that the
reagents might not perform as described in their manuals, we thoroughly monitor the accuracy of our daily tests. We are also monitoring the mutations, especially for the nucleic acid sequences of SARS-CoV-2 which are detected
by our PCR primers and probes.
For antigen test, various third-party research groups have evaluated our products. The reports suggest that our rapid antigen test (ESPLINE SARS-CoV-2 Ag) has high clinical sensitivity
in highly infectious samples from SARS-CoV-2 isolates and several samples from patients with COVID-19; it also shows the highest level of sensitivity among nineteen products using cultured viruses and demonstrates a clinical
sensitivity of 78.6% and clinical sensitivity with high viral load (Ct ≤25) of 92%. The high-sensitivity quantitative antigen test (LUMIPULSE SARS-CoV-2 Ag) is a more sensitive test than the rapid antigen test. It has been
reported that the sensitivity is comparable to that of other companies’ products. Our product is the only one that has been approved to use saliva samples, thereby reducing the risk of infection to healthcare professionals
during sample collection, as well as potentially minimizing the burden on medical facilities.
Various antibody tests, including IgM antibody test to detect early infection and IgG antibody test for spike protein, are
available to determine the effectiveness of vaccines for research purposes.
Refer to the website of FUJIREBIO Inc. for more details of the rapid antigen test.
A
PCR test detects genetic regions that do not easily mutate. In addition, variants are detectable by targeting multiple regions for amplification to minimize the effect of mutation. The antigen test detects nucleocapsid proteins, not spike proteins that are important in viral infections currently causing issues with occurrence of variants, and we use multiple antibodies that bind to different positions. We have confirmed that our product detects nucleocapsid proteins of the current variants of concern that are spreading infection. In addition to its ingenious design using several different types of antibodies to reduce the effect of mutations, we also monitor the trend of mutations on a daily basis and perform verification and confirmation tests when new mutations are reported.
A
Ct is defined as the number of cycles required for the fluorescent signal to cross the threshold to be positive. Ct value is inversely proportional to the amount of target nucleic acid in the sample; a lower Ct value means a greater amount of target nucleic acid in the sample, and a higher Ct value means a lower amount of target nucleic acid in the sample. Some say, “Higher Ct value means low infectivity/slightly positive.” However, Ct values should be interpreted with caution because Ct values may vary depending on the overall testing process, including sample collection, pretreatment, analytical method, technique, and combination of reagents and systems. Since numerous factors influence the Ct values, American Association for Clinical Chemistry recommends not to report or disclose Ct values for patient management. It is also important to note that all PCR reagents currently available are for “qualitative” tests and not designed for quantitative use (as of July 12, 2021). In addition, LAMP and TMA tests, classified as nucleic acid detection similar to PCR test, cannot obtain Ct values due to their measurement principles. Caution is needed as Ct values are not standardized because they vary depending on reagents and measurement systems.
Publications related to our products
Types of tests
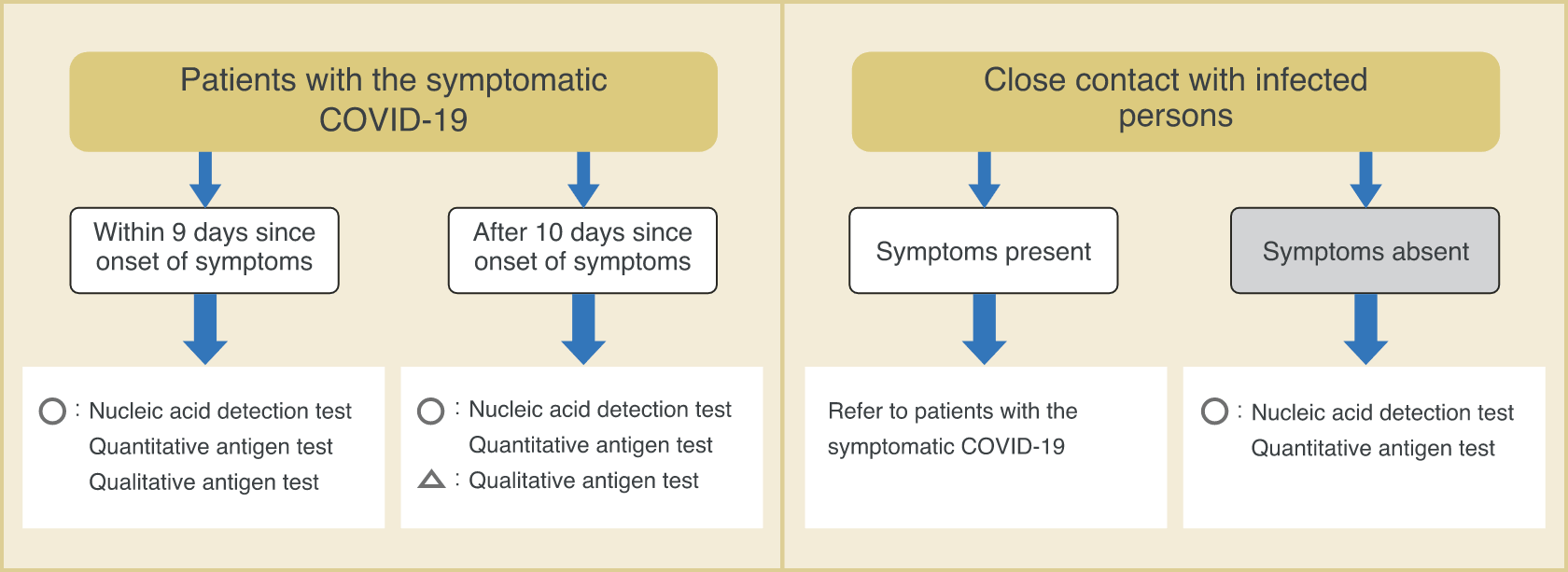
Ministry of Health, Labour and Welfare, et al. New Coronavirus Infectious Disease (COVID-19) Pathogen Testing Guideline Version 4
Targets
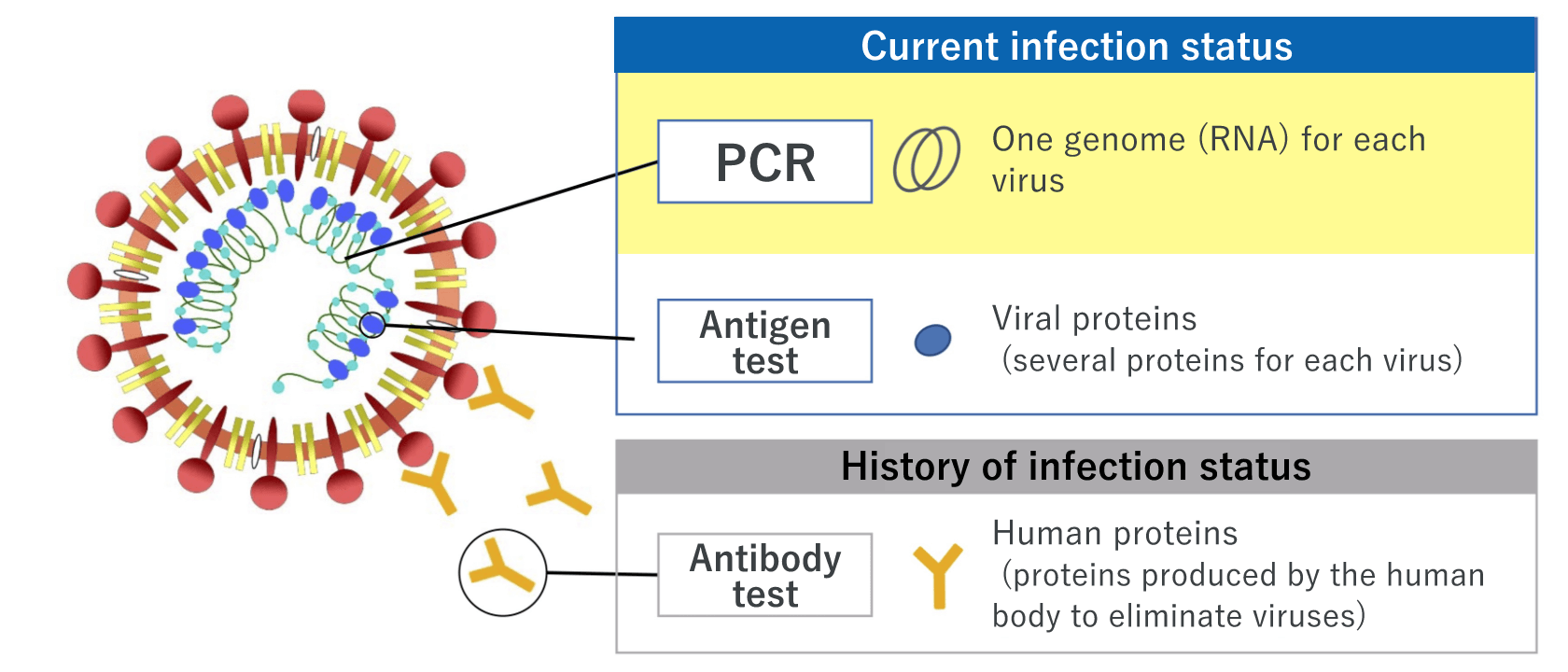
Targets
Targets